Endothermic Reaction Respiration . The reaction between ethanoic acid and sodium carbonate. Glucose is broken down in. some examples of endothermic reactions are: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. — for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. — in this way, cellular respiration is an example of energy coupling: endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to.
from classnotes123.com
revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. Glucose is broken down in. some examples of endothermic reactions are: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. — in this way, cellular respiration is an example of energy coupling: for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. The reaction between ethanoic acid and sodium carbonate. — for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products.
What does one mean by exothermic and endothermic reactions? Give
Endothermic Reaction Respiration The reaction between ethanoic acid and sodium carbonate. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. Glucose is broken down in. — for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. — in this way, cellular respiration is an example of energy coupling: The reaction between ethanoic acid and sodium carbonate. some examples of endothermic reactions are:
From wiringdbdementiushl.z4.web.core.windows.net
Endothermic And Exothermic Reaction Diagram Endothermic Reaction Respiration revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. — in this way, cellular respiration is an example of energy coupling: some examples of endothermic reactions are: Glucose is broken down in. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the. Endothermic Reaction Respiration.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Reaction Respiration for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. some examples of endothermic reactions are: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products.. Endothermic Reaction Respiration.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Respiration some examples of endothermic reactions are: revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products.. Endothermic Reaction Respiration.
From brainly.in
Show that photosynthesis is endothermic and respiration is exothermic Endothermic Reaction Respiration for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. — for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. some examples of endothermic reactions are: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. The. Endothermic Reaction Respiration.
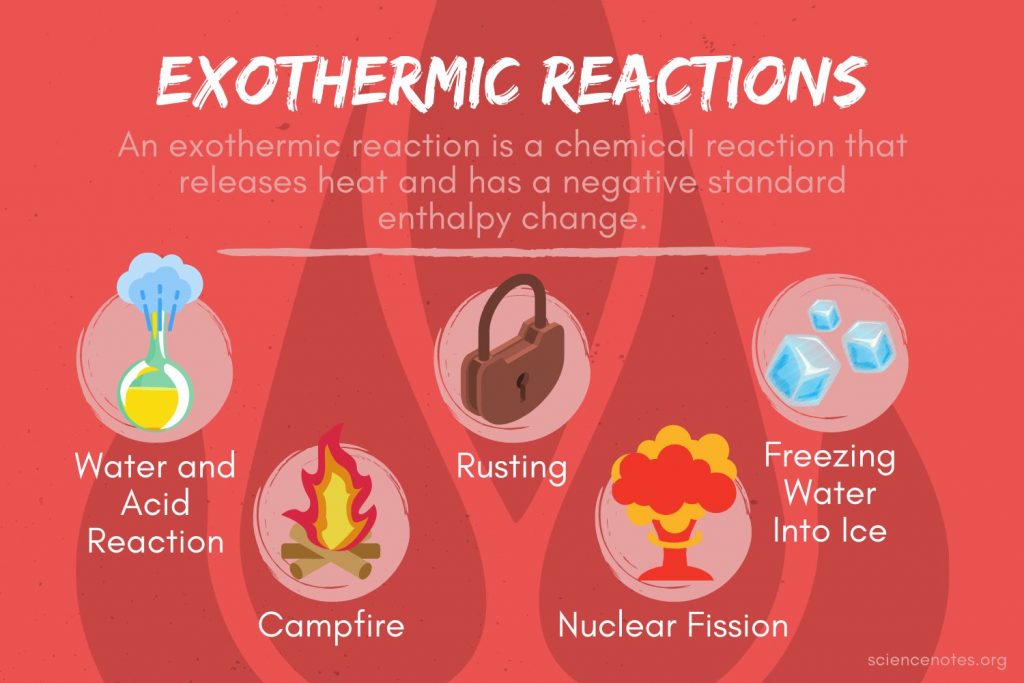
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Respiration Glucose is broken down in. — for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. The reaction between ethanoic acid and sodium carbonate. revise and understand what endothermic and exothermic reactions. Endothermic Reaction Respiration.
From slideplayer.com
Exothermic and Endothermic Reactions Oxidation Reactions ppt download Endothermic Reaction Respiration Glucose is broken down in. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. — for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. The reaction between ethanoic acid and sodium carbonate. for an endothermic chemical reaction to proceed, the reactants. Endothermic Reaction Respiration.
From stock.adobe.com
Endothermic reactions list with external energy outline collection set Endothermic Reaction Respiration — for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. The reaction between ethanoic acid and sodium carbonate.. Endothermic Reaction Respiration.
From kdi-ppi.com
A Closer Look at the Reaction Energy Diagram for Endothermic Reactions Endothermic Reaction Respiration These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. Glucose is broken down in. — for an endothermic chemical. Endothermic Reaction Respiration.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Respiration The reaction between ethanoic acid and sodium carbonate. — in this way, cellular respiration is an example of energy coupling: endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Glucose is broken down in. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their. Endothermic Reaction Respiration.
From slimpixs.blogspot.com
Endothermic And Exothermic Reactions Endothermic Reaction Examples Endothermic Reaction Respiration revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. some examples of endothermic reactions are: — for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. . Endothermic Reaction Respiration.
From www.youtube.com
Is Respiration Endothermic or Exothermic? YouTube Endothermic Reaction Respiration The reaction between ethanoic acid and sodium carbonate. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. Glucose is broken down in. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. some examples of endothermic reactions are: . Endothermic Reaction Respiration.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Respiration — for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. some examples of endothermic reactions are: endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Glucose is broken down in. The reaction between ethanoic acid and sodium carbonate. for an. Endothermic Reaction Respiration.
From slideplayer.com
1 Explain the role of the chloroplast. ppt download Endothermic Reaction Respiration Glucose is broken down in. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. — in this way, cellular respiration is an example of energy coupling: — for an endothermic chemical reaction to proceed,. Endothermic Reaction Respiration.
From www.flexiprep.com
NCERT Class X Science Solutions Chapter 1 Chemical Reactions and Endothermic Reaction Respiration some examples of endothermic reactions are: Glucose is broken down in. — in this way, cellular respiration is an example of energy coupling: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. — for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. for an. Endothermic Reaction Respiration.
From mavink.com
Endothermic Reaction Pathway Diagram Endothermic Reaction Respiration Glucose is broken down in. some examples of endothermic reactions are: — in this way, cellular respiration is an example of energy coupling: The reaction between ethanoic acid and sodium carbonate. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. These reactions lower the temperature of their. Endothermic Reaction Respiration.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Endothermic Reaction Respiration Glucose is broken down in. some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. — in this way, cellular respiration is an example of energy coupling: — for an endothermic chemical reaction. Endothermic Reaction Respiration.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Endothermic Reaction Respiration The reaction between ethanoic acid and sodium carbonate. — in this way, cellular respiration is an example of energy coupling: endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. — for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. These reactions. Endothermic Reaction Respiration.
From georgeilrussell.blogspot.com
Is Respiration Endothermic or Exothermic Endothermic Reaction Respiration some examples of endothermic reactions are: revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. — in this way, cellular respiration is an example of energy coupling: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions are chemical reactions. Endothermic Reaction Respiration.